Why non-coding RNAs?
During their life-span neurons undergo profound changes in gene expression that trigger rapid transitions in their developmental trajectory or functional state. The overarching goal of the research in the Lippi lab is to identify the key molecular players that drive and coordinate these transcriptional changes. In particular, we focus on non-coding RNAs (ncRNAs), a novel and exciting class of master regulators of gene expression that emerged during evolution to confer robustness to biological systems. Intriguingly, recent discoveries have suggested that the protein-coding repertoire has not changed much across multicellular organisms, and that increases in biological complexity mostly derives from the expansion of the ncRNA repertoire (Figure 1)
Figure 1. Relationship between biological complexity (increasing number of different cell types) and genetic information (protein-coding or non-coding genes). The number of protein-coding genes for each organism (orange squares) has plateaued at the dawn of multicellularity: there is little or no increase from Dictyostelium to H. Sapiens. The number of non-coding genes (blue rhombi), instead, shows a remarkable linear relationship with the number of different cell types. This suggests that to achieve such a massive increase in biological complexity, evolution did not add more building blocks (proteins), but layers over layers of sophisticated regulation of gene expression. Adapted from Liu, Cell Cycle 2013.
Many ncRNAs are enriched in the brain and increase their expression during development, suggesting that they play fundamental roles in establishing properly balanced neural networks. Consistently, a wealth of recent literature indicates that changes in ncRNA levels are linked to multiple neurodevelopmental disorders, including autism, schizophrenia and epilepsy. However, this is a brand new field and very little is known about how ncRNAs instruct network development.
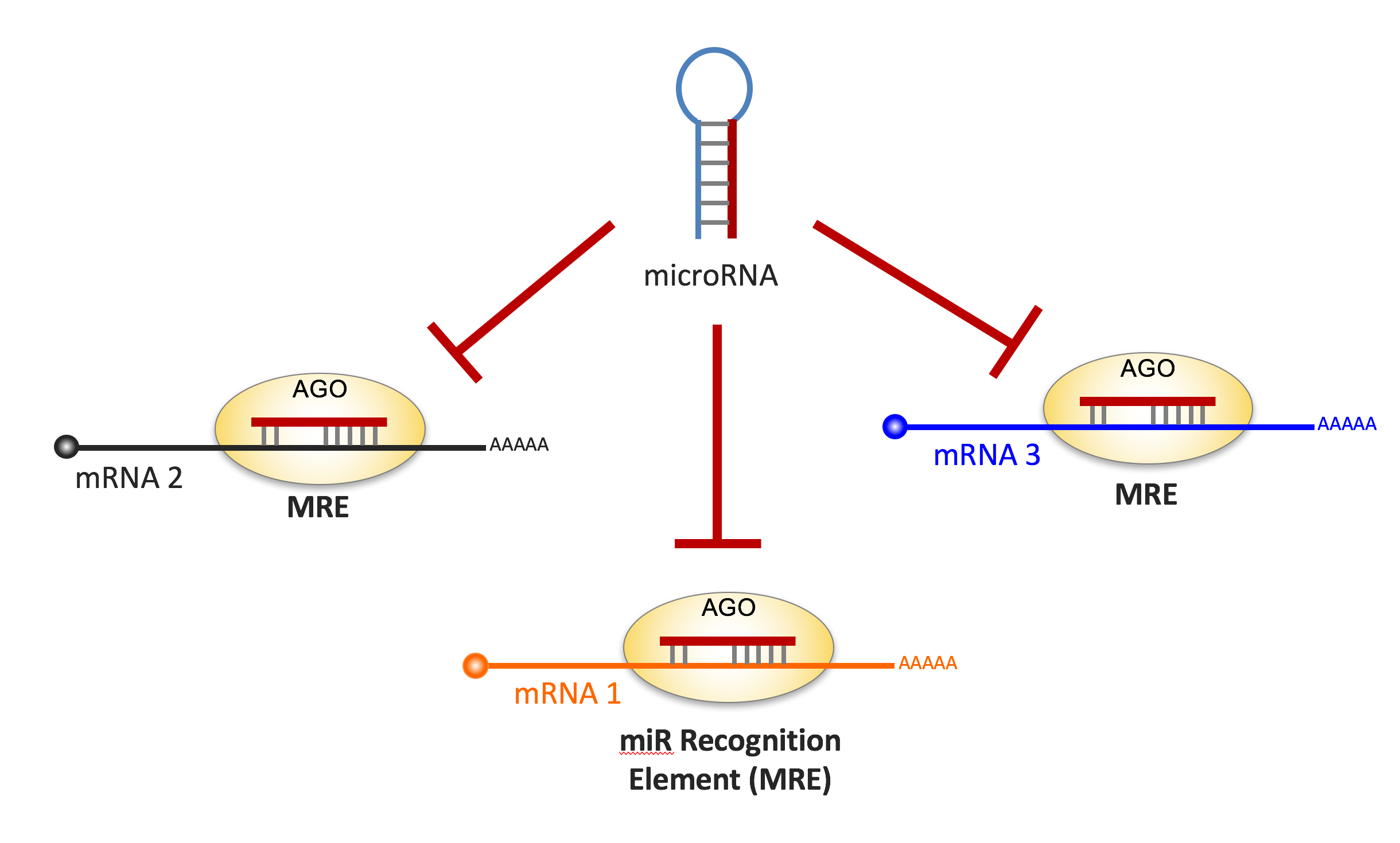
One of the most interesting class of ncRNAs is microRNAs (miRs). MiRs are post-transcriptional regulators of gene expression that bind and repress messenger RNAs (mRNAs) containing a miR recognition element (MRE). A single miR can target hundreds of different mRNAs, orchestrating epigenetic regulation of large combinations of gene products (Figure 2).
Figure 2. In their mature form miRs are loaded in the Ago complex, where they recognize mRNA targets based on sequence complementarity to a miR Recognition Element (MRE). Importantly, each miR can recognize hundreds of targets therefore consolidating specific gene programs.
The main theme of our work has been to understand how changes in miR levels mediate various forms of neuronal plasticity, including changes in structure (Lippi, JCB 2011), synaptic strength (Saba, MCB 2012), and neurotransmitter identity (Dulcis*, Lippi*, Neuron 2017). More recently, we have discovered that miRs are master regulators of the complex series of developmental events that determine neural network stability in the adult (Lippi, Neuron 2016). To do so we established a pipeline for a comprehensive analysis that identifies the function of miRs in vivo at multiple mechanistic levels, from molecular targets up through neuronal structure, network activity in freely behaving animals, and behavior. This is a novel role for a miR, and it has opened entire new avenues of research that have tremendous impact for both basic and translational neuroscience.
For more information please check the PUBLICATIONS and NEWS pages.